Original Articles
IL-6 inhibition improves nitric oxide in rheumatoid arthritis
Jaspreet Kaur1, Ashit Syngle2 *, Pawan Krishan1, Kanchan Vohra1, Nidhi Garg1, Ladbans Kaur3, Monica Chhabra4
Author Affiliations
1Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala, Punjab, India
2*Managing Director, Cardio-Rheuma & Healing Touch City Clinic, Chandigarh & Senior Consultant Physician and Rheumatologist, Fortis Hospital, Mohali, Punjab, India
3Consultant Radiologist, Prime Diagnostics, Chandigarh, India
4Consultant Radiologist, Fortis Multispecialty Hospital, Mohali, Punjab, India
*Correspondence: Dr. Ashit Syngle
IJRCI. 2014;2(1):OA2
Received: 8 February 2014, Accepted: 2 May 2014, Published: 27 May 2014
© IJRCI
Abstract
Aim
To investigate the serum nitrite levels in patients with active rheumatoid arthritis (RA) after treatment with tocilizumab, an IL-6 antagonist, and to correlate between inflammatory disease activity measures and endothelial dysfunction.
Materials and methods
Eleven anti-IL-6-naive RA patients with high disease activity (DAS28 >5.1) were investigated. Inflammatory disease activity (DAS28 and HAQ-DI scores, ESR and CRP), serum nitrite concentration and flow mediated dilation (FMD) of the brachial artery were measured before and after 12 weeks of therapy with tocilizumab 8mg/kg intravenous infusion every 4 weeks. Serum nitrite levels and FMD were also estimated in 10 age- and sex- matched healthy controls.
Results
Serum nitrite levels in RA patients at baseline were significantly higher when compared to healthy volunteers (P <0.01). Level of serum nitrite significantly decreased (P <0.001), inflammatory disease activity measures and FMD significantly improved (P <0.001) after treatment. There was a significant correlation of serum nitrite with FMD, CRP, and DAS 28 both at baseline (P <0.05) and after 12 weeks therapy (P <0.05).
Conclusion
Nitrite production is enhanced to a greater extent in patients with RA compared to controls. Reduction in nitrite level after treatment is associated with the improvement in disease activity and endothelial dysfunction. Serum nitrite level may serve as a good predictor of both inflammatory disease activity and response to therapy in RA.
Introduction
Association between rheumatoid arthritis (RA) and atherosclerotic vascular disease is well recognized and these patients have 4-fold increased risk of cardiovascular disease (CVD).1 In fact, RA patients die prematurely and CVD is the major cause of excessive mortality.2 Traditional cardiovascular (CV) risk factors do not adequately account for the extent of CVD in RA.3
Inflammatory cytokines (TNF-α, IL-1, and IL-6) released from the synovium in RA patients generate a spectrum of proatherogenic changes such as insulin resistance, dyslipidemia, oxidation, upregulation of cellular adhesion molecule expression on endothelial cells, thrombin generation and platelet activation. These in turn contribute to an accelerated atherosclerosis.4 In addition, patients with RA have a dual abnormality in nitric oxide (NO)-dependent vascular function, characterized by blunted endothelial nitric oxide synthase (eNOS) and enhanced inducible nitric oxide synthase (iNOS) by leukocytes and vascular smooth muscle cells (VSMCs).1 Endothelial cells (ECs) serve as major targets for these cytokines where they induce the expression of iNOS and produce excessive amount of NO. Consequently, NO reacts with superoxide to form the toxic derivative peroxynitrite (ONOO-) that destroys endothelin layer by nitration of proteins and causes endothelial dysfunction.5
IL-6 is the most abundant cytokine in RA and increases iNOS expression through gp130 signaling pathway.6 Patients with high levels of IL-6 are 2-5 times more likely to have myocardial infarction, stroke, and other CV events, and specific evidence-based recommendations exist for CV risk management of RA patients.7, 8
NO plays a central role in the regulation of blood vessel tone and inflammation. The effect of infliximab, and biologic DMARDs on NO in RA has been studied recently, but the effect of IL-6 blockade on NO has not been investigated.9 In view of this, we have studied the impact of IL-6 blockade with the humanized recombinant monoclonal antibody, tocilizumab, on NO and its correlation with inflammation and vascular function in RA patients with high disease activity, who were on conventional DMARDs.
Patients and methods
The study protocol was approved by the regional ethical research committee and was performed in accordance with the declaration of Helsinki and the code of Good Clinical Practice. The investigators (two radiologists and one pharmacologist) involved in estimating the two critical components of the study - serum nitrite concentration and flow mediated vasodilatation (FMD) of brachial artery - were blinded to the treatment protocol. Other clinical investigations and assessments of participants were carried by the rheumatologist. All the patients provided written informed consent to participate after a full explanation of the study.
Fifteen patients (aged >18 years) were screened for definite RA, based on the 2010 Rheumatoid Arthritis Classification Criteria for diagnosis and classification of RA.10 One patient had latent tuberculosis and three patients withdrew their consent to participate in the study. Despite treatment with single or combination conventional DMARDs, eleven patients with definite RA (all women; mean age 51.6±1.4 years) with mean disease duration of 11.36 ± 2.58 years and high disease activity were enrolled in the study from a rheumatology outpatient clinic. All the patients included were having active RA, defined by the presence of modified DAS28 score ≥ 5.1, presence of six or more tender joint count (TJC) and swollen joint count (SJC), and at least two or more of the following: morning stiffness that lasted for at least 30 minutes and erythrocyte sedimentation rate (ESR) ≥28 mm in 1st hr or serum CRP >2 mg/dl. Patients had to be taking stable dose of DMARDs for at least 3 months before entering the study. Eligible patients were on stable doses of DMARDs [methotrexate (15 mg/wk), hydroxychloroquine (400 mg/day), sulfasalazine (2-3 g/day) or leflunomide (20 mg/day)] for at least 3 months before entering the study. Oral glucocorticoids (≤10 mg/day prednisone or equivalent) were permitted, if the doses were stable for 6 weeks. All the subjects were teetotallers and nonsmokers, who were on regular normal diet.
Exclusion criteria were: age <18 years, history of acute inflammatory joint disease other than RA, prior use of biologics, platelet count <1,00,000/mm3, serum creatinine level >2 mg/dl, persistently abnormal liver function tests (alanine aminotransaminase (ALT)/aspartate aminotransaminase (AST) >2-fold the upper normal limits), active hepatitis, HIV infection, active tuberculosis, multiple sclerosis, live vaccine administration, insulin dependent diabetes mellitus, uncontrolled type 2 diabetes mellitus (HbA1c >7.0) and hypertension (>160/90 mmHg), history of hyperlipidemia, pregnant and lactating women, active inflammatory bowel or peptic ulcer disease, drug and alcohol abuse, concomitant use of drugs known to affect endothelial function (nitrates, statins, angiotensin converting enzyme inhibitors, angiotensin receptor blockers and beta-blockers), and history of sensitivity to the study medication.11 Individuals with CVD, renal disease and current smokers were also excluded because these conditions are associated with endothelial dysfunction and arterial stiffening. Serum nitrite levels and flow-mediated dilation (FMD) were also estimated in 10 age- and sex- matched healthy controls.
All the patients continued their DMARD regimen and added tocilizumab at a dose of 8 mg/kg every 4 weeks, as a 60 minute intravenous infusion. Patients were monitored for 24 h after infusion, for possible adverse effects.
Assessment
In all subjects, the following hematological and other laboratory tests were determined by established protocol using standard kits: hemoglobin, total leucocyte count, differential leucocyte count, platelet count, serum creatinine, liver enzymes, serum uric acid, lipid profile, ESR, and CRP.
Disease activity and inflammation
Clinical evaluation of inflammation in terms of ESR and CRP levels, and disease activity using DAS28 ESR (4) were performed at baseline and after 12 weeks.12 The Indian version of Health Assessment Questionnaire-Disability Index (HAQ-DI) completed by each subject was used to assess their disease activity and functional capability.13
Serum nitrite concentration
Nitric oxide was directly determined as nitrite by a spectrophotometric method, using serum samples collected after at least 14 h of fasting.14-16 Gaseous NO free radical is rapidly metabolized to nitrate or nitrite, hence measured nitrite concentration is taken as an index of NO production.14 Nitrite is also not influenced by dietary variation.15
All the subjects were teetotallers and nonsmokers and taking a normal regular diet. Subjects were asked to fast after the evening meal at 6.00 pm till 8.00 am next morning, when the sample was obtained.17 Samples were estimated in duplicate and mean of two estimations was considered for further assessments.
Assessment of endothelial function
Flow-mediated vasodilation and nitroglycerin-induced (400 µg sublingual tablet) vasodilation of the brachial artery were determined by standardized method using a high-resolution ultrasound vessel wall tracking device with a 10-MHz linear-array transducer (Voluson E6 BT10 version; GE, Zalsburg, Austria), as described in earlier studies.18 Two radiologists involved in FMD determinations were blinded to the treatment protocol. FMD was performed a day before the scheduled dose of biologic treatment. Subjects were asked to fast without water, for at least 12 h prior to being assessed. Subjects lay supine for 20 min before the first vascular ultrasound scan was conducted. FMD of the brachial artery was induced by release of a wrist cuff inflated to supra-systolic pressure for 5 min. Recording of arterial diameter commenced 30 sec before cuff deflation, and continued for 3 min with recordings at every 15 sec. After a rest period of 10 min, when brachial artery diameter had returned to baseline levels, a second baseline scan was recorded. This was followed by sublingual administration of nitroglycerin and the diameter was recorded every 30 sec for 5 min.18 FMD was assessed at baseline and after 12 weeks of study treatment. FMD was calculated as:
FMD (%) = 100 x (post-hyperemia diameter – basal diameter)/basal diameter
Statistical analysis
Results are presented as mean ± SEM. Differences in parameters before and after tocilizumab treatment were examined by 2-tailed paired student t-test. Measurement of FMD represents the maximal increase in brachial artery diameter and is expressed as percent change from baseline. Univariate correlation analysis was used to examine the relationship between serum nitrite concentration, FMD, DAS28 and CRP at baseline and after therapy. Statistical significance was accepted at P ≤0.05.
Results
Patient profile: The baseline demographic and clinical characteristics of the patients and controls are presented in table 1, and patient characteristics documented before and after therapy are presented in table 2.
Table 1: The demographic and clinical characteristics of the patients and controls at baseline
Table 2: Patients characteristics before and after the treatment
Tocilizumab improves serum nitrite concentration
In healthy volunteers, mean serum nitrite level was 5.0±0.11 µmol/L. Serum nitrite levels were higher in RA patients when compared to healthy controls (P <0.01) at baseline (Table 1); and decreased from 7.41±0.18 to 6.13 ±0.19 (P <0.001) (Table 2) after 12 weeks of treatment with tocilizumab (Fig.1).
Tocilizumab improves vascular function
Endothelial-dependent FMD improved significantly from 8.21±0.88 to 14.60±0.96 after 12 weeks of treatment with tocilizumab (P <0.001), while endothelial-independent vasodilation induced by nitroglycerine remained unchanged (19.47±0.99% vs. 20.06±0.74%; P = 0.50). There was no significant difference in brachial artery diameter from baseline to end visits (2.78 ± 0.12 vs. 2.88 mm ± 0.16; P = 0.25) (Table 2). FMD at baseline was significantly impaired in RA patients when compared to matched controls (8.21±0.88 vs. 20.0 ± 1.2, P <0.001). In addition, mean baseline diameter for the groups did not differ significantly (2.78±0.12 vs. 2.98±0.19 mm, P >0.05) (Table 1).
Tocilizumab improves inflammatory disease activity
All of the subjects had high disease activity, as reflected by DAS28 score ≥5.1. After 12 weeks of treatment, disease activity of nine patients had fallen to moderate levels (3.2 to 5.1), that for one patient achieved low (≤3.2) while for another, it was maintained at a high disease activity. Overall, a composite DAS28 score improved significantly from 6.59 ± 0.24 to 4.47 ± 0.24 (P = 0.005) after treatment (Table 2).
HAQ-DI scores decreased significantly from 2.17 ± 0.14 to 1.08 ± 0.97 (P <0.001) (Table 2). Consistently, ESR decreased significantly from 57.45 ± 8.9 to 25.27 ± 5.11 mm in the first hour (P = 0.005) and CRP level decreased from 34.87 ± 8.01 to 13.38 ± 3.60 mg/dL (P = 0.004) after treatment (Table 2).
Association of serum nitrite with inflammatory disease activity and endothelial function
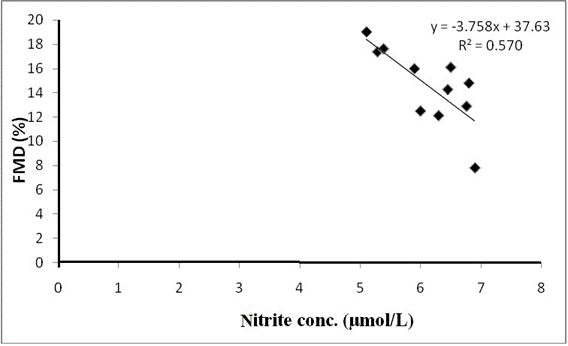
At univariate analysis, significant positive correlation was observed for serum nitrite with DAS28 (r = 0.715, P = 0.011*) and CRP (r = 0.745, P = 0.008*) at baseline (Table 3); and significant negative correlation was observed for serum nitrite with FMD (r = -0.683, P =0.020*) at baseline (Table 3). After therapy, significant positive correlations of serum nitrite with DAS28 (r = 0.652, P = 0.010*) (Fig. 2A) and CRP (r = 0.710, P = 0.014*) were sustained, so also the negative correlation with FMD (r = -0.755, P = 0.007*) (Fig.2B) as indicated in table 3.
Safety profile
Tocilizumab did not produce any significant change in hematological and biochemical profile of the patients. None of the patients reported any serious adverse effect. One patient reported gastroenteritis and another upper respiratory tract infection during the study period, which were managed conservatively.
Table 3: Univariate analysis of serum nitrite with various selected variables visits
Fig.1: Effect of tocilizumab on nitrite concentration after treatment
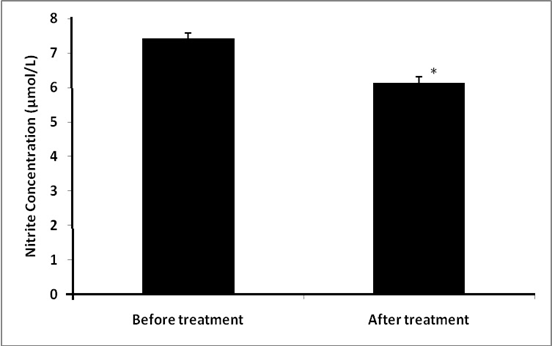
Nitrite concentration improved significantly from 7.41±0.18 to 6.13 ±0.19 (p<0.001) after 12 weeks of initial treatment.
* Significance P <0.05
Fig.2A: Correlation between nitrite concentration and DAS-28 after treatment
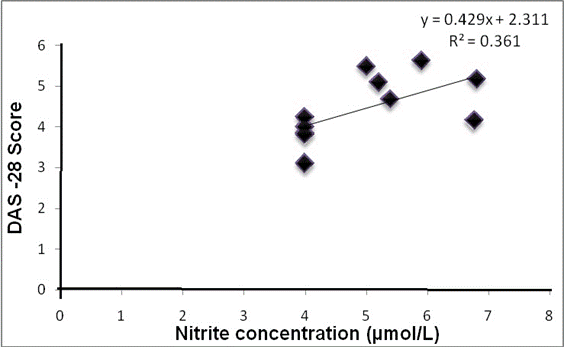
Fig.2B: Correlation between nitrite concentration and flow mediated dilation (FMD) after treatment
Discussion
This is the first study to investigate the impact of tocilizumab on NO, in biologic-naïve RA patients. NO has an important role in the pathogenesis of RA and acts as a key mediator of apoptosis within the RA joints. It stimulates a variety of pro-inflammatory cytokines activated through iNOS.19 RA is characterized by an increased nitrite concentration and nitric oxide synthase inhibition improves the inflammatory disease activity. IL-6 along with other inflammatory mediators decrease endothelial nitric oxide synthase (eNOS) expression, in part, by activating STAT3 binding to the eNOS 5’ flanking region, thereby inducing the transcription of acute phase response reactants such as CRP. These reactants contribute to the development and progression of CVD by reducing NO production or response to NO.20 Cytokines, including IL-6, enhance iNOS expression on vascular smooth muscle cells, leading to production of large amount of NO, but the response to NO is decreased.21 In an oxidative environment, NO damages vascular endothelium through nitration of proteins. Although tocilizumab is a humanized anti IL-6 receptor antibody with impressive effects on musculoskeletal inflammation in RA, it impacts nitric oxide synthases as well as it blocks the activation of iNOS by interfering with the gp130 signaling pathway and thus inhibiting NO release.6
IL-6 upregulates AT1R gene expression and may lead to increased angiotensin II (Ang II)-mediated vasoconstriction and reactive oxygen species (ROS) production, in turn leading to endothelial dysfunction.22 Moreover, it has been suggested in some studies, that the effect of Ang-II on endothelial function is attributable to O2 mediated inactivation of NO.23, 24 Additionally, IL-6 expression may be an important link between Ang II- induced increase in NAD(P)H oxidase activity, thereby limiting the bioavailability of NO for normal vascular responses.25 Therefore, NO may prove to be an important therapeutic target in inflammatory diseases like RA, that has not yet been investigated with tocilizumab.
The current study subjects, with active RA disease and being biologic-naïve, exhibited increased serum nitrite concentration at baseline. These results are in accordance with previous studies, and attributed to increased iNOS expression and enhanced NO formation.26, 27 iNOS activity is very low in normal conditions and is stimulated during inflammation by cytokines, such as IL-6, and the amount of NO produced by iNOS may be 1,000-fold greater than that produced by endothelial NOS, leading to local (synovial fluid) and systemic increase in nitrite and nitrate concentrations. Thus, depending on the concentration of NO, it can act as a pro- or anti-inflammatory agent.6 Elevated nitrite levels associated with an active disease suggest that enhanced NO formation may be correlated with enhanced inflammatory disease activity in RA. 28 After 3 months of treatment with tocilizumab, the significant reduction in serum nitrite concentration (P <0.001) suggests that IL-6 blockade also has a beneficial impact on NO in active RA, similar to TNF-α blockade.6
A significant improvement in clinical assessment of DAS28 (P <0.001) suggests reduction in inflammatory disease activity. The improvement in DAS28 with tocilizumab was similar to the study results of ‘OPTION’.29 Moreover, significant positive correlation of serum nitrite with DAS28 (P = 0.01) after therapy suggested that decrease in serum nitrite level parallels decrease in disease activity. All the patients included in the present study had high disease activity (DAS28 >5.1) and demonstrated an improvement to moderate severity (3.2 to 5.1) after treatment with tocilizumab. Moreover, the compliance of the efficacy results for tocilizumab with previously published ADACTA study adds credibility to the present study design and findings.30
CRP contributes directly to the pathogenesis of endothelial dysfunction and atherosclerosis. It has been shown to be associated with CVD in rheumatoid arthritis, as it decreases eNOS expression by destabilizing its mRNA, and increases the expression of endothelial adhesion molecules, like ICAM, VCAM, E-selectin and MCP-1.32, 33 Significant reduction in CRP and ESR noted in the present study reflects a reduction in the severity of systemic inflammation after treatment with tocilizumab. Elevated serum CRP levels may be due to an increased production of IL-6 induced by the activated monocytes, and tocilizumab is known to inhibit the action of IL-6, thereby reducing the production of acute phase proteins, such as CRP and fibrinogen from hepatocytes.31 A significant correlation of serum nitrite with CRP at baseline (P <0.05), and after treatment (P <0.05) suggests decreased iNOS expression and formation of NO that signals a reduction in systemic inflammation.
A blunted vasodilator response to FMD was also noted for the treatment group compared to the control group. Inflammation has the potential to impair FMD and the observed blunted response implies a decrease in NO bioavailability (due to decreased expression of eNOS) and this may lead to an elevated CVD risk due to accelerated atherosclerosis. A decrease in FMD could be independently predicted by increased ESR and inflammatory DAS28. Increased production of NO in RA due to the activation of iNOS and an attenuation of eNOS activity reduces basal FMD. This argument supports the observation that IL-6 blockade has also shown significant (P <0.001) improvement in vascular endothelial function of the brachial artery, in addition to disease amelioration as evidenced by reduction of CRP, ESR, and DAS28 scores. We also found a significant negative correlation between nitrite and FMD (r = -0.683), P = 0.020). This is in line with previous studies suggesting that there is decreased iNOS expression and NO production through increase in eNOS, which has favorable effect on FMD.27, 34
In the current study, subjects demonstrated FMD in the range of 7-9%, signifying a moderate risk of CVD. The improvement in FMD (14.6%) noted after 3 months of therapy with tocilizumab decreased the CVD risk. In a recent study, the subjects had a baseline FMD of 3.3% signifying high CVD risk, which subsequently increased to 4.4% and 5.2% after 3 and 6 months of treatment, respectively. However, the study findings indicated that these patients had high CV risk, despite improvement in FMD.35 In the present study, improvement in FMD could also have resulted from the improvement in general inflammatory state that characterizes RA. The significant increase in the FMD response, but not the NTG response, suggests that after treatment with tocilizumab, there is an increase in NO bioavailability, but not the sensitivity of vascular smooth muscle to NO.
A small but non-significant increase in total cholesterol and triglycerides observed in the treatment group, compiles with the previous data on tocilizumab, showing that the increase may be due to anti-IL6 treatment.31, 36 This may be secondary to the improvement in inflammation. Furthermore, no clinical symptoms or CV events associated with increase in total cholesterol were reported. In contrast to previous study findings, a marginal, though non-significant, increase in HDL- and reduction in LDL-cholesterol was observed in the present study.
The HAQ-DI is a standard and validated instrument for self-assessment of health status in RA. Previous studies have shown that patients treated with tocilizumab had a clinically meaningful improvement in physical function, and the current observations are consistent as indicated by a significantly improved HAQ-DI (P <0.01), reflecting substantial functional benefits for the patients.37
Two patients reported adverse events during the study period – one had an episode of upper respiratory tract infection and the other had gastroenteritis. The severity of adverse events is consistent with that described in previous reports, but the incidence rate is relatively low, possibly due to small number of patients and shorter duration of the study.36, 37
The present study, a pioneering one to the best of our knowledge, has demonstrated a significant decrease in serum nitrite concentration. Although the magnitudes may appear to be small, majority of the treated subjects had moderate disease activity after 12 weeks of treatment with tocilizumab. However, small sample size, shorter duration, and not having a placebo arm are the limitations of the study. Serum nitrite is a better marker for detection of endothelial dysfunction than other inflammatory markers. The impairment in NO balance is the underlying cause for endothelial dysfunction, the main cause of CVD in RA. Reduced serum nitrite concentration after treatment with tocilizumab, and consequent improvement in inflammatory disease activity and endothelial dysfunction provides a promising strategy to manage RA and its complications.
Competing interests
The authors declare that they have no competing interests.
References
1. Van Leuven SI, Franssen R, Kastelein JJ, Levi M, Stroes ESG, Tak PP. Systemic inflammation as a risk factor for atherothrombosis. Rheumatology (Oxford). 2008 Jan;47(1):3–7.
2. Taylor PC. Serum vascular markers and vascular imaging in assessment of rheumatoid arthritis disease activity and response to therapy. Rheumatology. 2005 Jun 1;44(6):721–8.
3. Del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001 Dec;44(12):2737–45.
4. Sattar N, McCarey DW, Capell H, McInnes IB. Explaining How “High-Grade” Systemic Inflammation Accelerates Vascular Risk in Rheumatoid Arthritis. Circulation. 2003 Dec 16;108(24):2957–63.
5. Endemann DH, Schiffrin EL. Endothelial Dysfunction. JASN. 2004 Aug 1;15(8):1983–92.
6. FischerP, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: The gp130-STAT3 axis. Basic Res. Cardiol. 2007;102:393–411.
7. Cesari M, Penninx BWJH, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003 Nov 11;108(19):2317–22.
8. Peters MJL, Symmons DPM, McCarey D, Dijkmans BAC, Nicola P, Kvien TK, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010 Feb;69(2):325–31.
9. Gonzalez-Gay MA, Garcia-Unzueta MT, Berja A, Vazquez-Rodriguez TR, Miranda-Filloy JA, Gonzalez-Juanatey C, et al. Short-term effect of anti-TNF-alpha therapy on nitric oxide production in patients with severe rheumatoid arthritis. Clin Exp Rheumatol. 2009 Jun;27(3):452–8.
10. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010 Sep 1;69(9):1580–8.
11. Koike R, Harigai M, Atsumi T, Amano K, Kawai S, Saito K, et al. Japan College of Rheumatology 2009 guidelines for the use of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, in rheumatoid arthritis. Mod Rheumatol. 2009;19(4):351–7.
12. Van Gestel AM, Prevoo ML, van ’t Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996 Jan;39(1):34–40.
13. Kumar A, Malaviya AN, Pandhi A, Singh R. Validation of an Indian version of the Health Assessment Questionnaire in patients with rheumatoid arthritis. Rheumatology (Oxford). 2002;41:1457–9.
14. ClancyMC, AminAR, AbramsonSB. The role of nitric oxide in inflammation and immunity.Arthritis Rheum.1998;41:1141-51.
15. Knight TM, Forman D, Al-Dabbagh SA, Doll R. Estimation of dietary intake of nitrate and nitrite in Great Britain. Food Chem Toxicol. 1987 Apr;25(4):277–85.
16. Sastry KVH, Moudgal RP, Mohan J, Tyagi JS, Rao GS. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal Biochem. 2002 Jul 1;306(1):79–82.
17. Himeno M, Ishibashi T, Nakano S, Furuya K, Kigoshi T, Uchida K, et al. A practical procedure for achieving a steady state of NOx concentration in plasma: with special reference to the NOx content of Japanese daily food. Tohoku J Exp Med. 2003 Feb;199(2):95–110.
18. Hürlimann D, Forster A, Noll G, Enseleit F, Chenevard R, Distler O, et al. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002 Oct 22;106(17):2184–7.
19. Nagy G, Koncz A, Telarico T, Fernandez D, Ersek B, Buzás E, et al. Central role of nitric oxide in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther. 2010;12(3):210.
20. Saura M, Zaragoza C, Bao C, Herranz B, Rodriguez-Puyol M, Lowenstein CJ. Stat3 Mediates Interelukin-6 Inhibition of Human Endothelial Nitric-oxide Synthase Expression. J Biol Chem. 2006 Oct 6;281(40):30057–62.
21. Vila E, Salaices M. Cytokines and Vascular reactivity in resistance arteries. Am. J. Physiol. Heart Circ. Physiol.2005;288: H1016-H1021.
22. Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Böhm M, et al. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res. 2004 Mar 5;94(4):534–41.
23. Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996 Apr 15;97(8):1916–23.
24. Didion SP, Kinzenbaw DA, Faraci FM. Critical role for CuZn-superoxide dismutase in preventing angiotensin II-induced endothelial dysfunction. Hypertension. 2005;46:1147-1153.
25. Hou T, Tieu BC, Ray S, Recinos III A, Cui R, Tilton RG, et al. Roles of IL-6-gp130 Signaling in Vascular Inflammation. Curr Cardiol Rev. 2008 Aug;4(3):179–92.
26. Ersoy Y, Ozerol E, Baysal O, Temel I, MacWalter RS, Meral U, et al. Serum nitrate and nitrite levels in patients with rheumatoid arthritis, ankylosing spondylitis, and osteoarthritis. Ann Rheum Dis. 2002 Jan;61(1):76–8.
27. Syngle A, Vohra K, Kaur L, Sharma S. Effect of spironolactone on endothelial dysfunction in rheumatoid arthritis.Scan J Rheumatol. 2009;38:15-22.
28. Farrell AJ, Blake DR, Palmer RM, Moncada S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases.Ann. Rheum. Dis.1992;51:1219-1222.
29. Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008 Mar 22;371(9617):987–97.
30. Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Pavelka K, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. The Lancet. 2013 May;381(9877):1541–50.
31. Nishimoto N, Yoshizaki K, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:1761–1769.
32. Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002 Sep 17;106(12):1439–41.
33. Pasceri V, Cheng JS, Willerson JT, Yeh ET, Chang J. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001 May 29;103(21):2531–4
34. Syngle A, Vohra K, Garg N, Kaur L, Chand P. Advanced glycation end products inhibition improves endothelial dysfunction in rheumatoid arthritis.Int J Rheum Dis. 2012;15:45–55.
35. Protogerou AD, Zampeli E, Fragiadaki K, Stamatelopoulos K, Papamichael C, Sfikakis PP. A pilot study of endothelial dysfunction and aortic stiffness after interleukin-6 receptor inhibition in rheumatoid arthritis. Atherosclerosis. 2011 Dec;219(2):734–6.
36. Nishimoto N, Hashimoto J, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007 Sep;66(9):1162–7.
37. Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008 Oct;58(10):2968–80.