Original Articles
Variation in pressure pain threshold correlates with rheumatoid arthritis disease activity, not with the inflammatory parameters
Arun MS1, Chandrashekara S2*, Suresh KP3
Author Affiliations
1Consultant Rheumatologist, Arushi Rheumatology Centre, Saadhanaa road, Tumkur, Karnataka, India
2*Managing Director and Consultant Rheumatologist, Chanre Rheumatology and Immunology Center, Basaweswaranagar, Bangalore, India
3Scientist (SS), Office Project Directorate on Animal Disease monitoring and Surveillance, Hebbal, Bangalore, India
*Correspondence: Dr. Chandrshekara S
IJRCI. 2014;2(1):OA1
Received: 2 February 2014, Accepted: 26 March 2014, Published: 5 April 2014
© IJRCI
Abstract
Background
Pain is a chief symptom of rheumatoid arthritis (RA) indicating the disease flare. Variation in pain sensitivity with inflammatory load may assist in measuring the disease activity. Tender joint counts, one of the currently used pain measures, is more a measure of active joints rather than the pain. Although visual analogue scale (VAS) reflects the perception of pain, it does not indicate the true change in the pain.
Aim
To evaluate whether pressure pain threshold (PPT) represents disease activity by comparing it with DAS-28 and inflammatory markers like ESR and CRP.
Materials and methods
Twenty freshly diagnosed female RA subjects were recruited after careful exclusion of patients with fibromyalgia (FM). They were assessed for tender joint count, swollen joint count, visual analogue scale (pain/general health), ESR, and CRP during their three consecutive follow-up of six week intervals. PPT was measured using digital algometer at three pairs of joint areas (metacarpophalangeals, wrists and most inflamed joint) and at three FM tender point sites (sub-occipital, mid-trapezius and buttocks). Arithmetic mean of all areas (average PPT) and average PPT of FM points were calculated. The data was analyzed using Pearson correlation co-efficient, Deming regression analysis and correlation of change.
Results
Average PPT and PPT of most inflamed joint have moderate to large correlation with DAS-ESR and DAS-CRP. In contrast, small to trivial correlation with ESR and CRP was noted. Similar results were seen for FM tender point sites.
Conclusion
PPT at inflamed as well as at FM sites correlates significantly with DAS-ESR and DAS-CRP. It does not correlate independently with changes in inflammatory parameters ESR and CRP. PPT in conjuncture with DAS could be useful in routine clinical practice to measure the pain as well disease activity, in RA patients.
Introduction
Although pain has been recognized as an important symptom indicating disease activity in rheumatoid arthritis (RA), its measurement is challenging. Currently, pain is measured on the basis of tender joint counts and visual analogue scale (VAS) indicating the patient’s perception of overall pain. The tender joint count measures pain by eliciting pressure tenderness in joints with active disease rather than the pain per se felt or experienced by the patients.1 Lack of uniformity in applying pressure is one of the major limitations of this method, as it may differ at different times in same patient, and also among different examiners. The adoption of simple thumb rule for applying pressure enough to cause blenching of thumb and training physicians for joint examination are used to standardize the technique of eliciting tenderness. However, the persistence of individual variation limits the method.2 The VAS, reflecting the pain perception may not indicate the absolute change in pain, as the scoring is based on the pain experienced by the patient in a specified period of time in comparison to the lifetime experience. Hence it is vulnerable to variation, especially when used in a group of patients.1, 3 In addition, the measures such as visual analogue scale-general health (VAS-GH), and tender joint count (TJC) (two-dimensionality of DAS28 score) exhibit lot of subjectivity.1 Lack of a direct measure of pain amongst the core set measures is one of the major drawbacks in RA pain management.
The pain being an important symptom of RA, quantifying pain in a patient should assist in assessing the disease activity. Both the American College of Rheumatology (ACR) response criteria and the Disease Activity Score (DAS), approved by European League Against Rheumatism Response Criteria (EULARC), recommend tender joint and VAS measure of disease activity as important core set criteria, in addition to joint counts. 2, 4
The pressure pain threshold (PPT), defined as the minimum pressure stimulus applied to elicit pain, could be suitable to measure pain in RA. PPT, which is independent of patient’s experience, will be more objective than other measures. Evidence indicate that alteration in pain sensitivity is proportional to inflammatory load and it is inversely related to CRP and cytokines like TNF-alpha and IL6.5, 6, 7, 8 PPT has been validated as a useful tool to measure pain, even in normal healthy individuals, as the measured values are reliable and highly consistent.9 Hence it could also function as a surrogate marker of disease activity in RA.
Based on the above facts, we hypothesize that the changes in PPT may represent the change in inflammatory load, which in turn correlate to DAS28. Since RA can affect one or all the 66 joints, it may be challenging to measure all these areas, though it can be closer to disease measure and reflects disease activity. The alteration in pain threshold in active RA occur both at the inflamed joints as well as in other sites.10 The changes are often reported to occur even at the designated tender points of fibromyalgia (FM).10 Monitoring and documenting disease activity in RA in daily practice should be easy to perform and not time consuming.11 Moreover, it should assist physicians in making treatment decision. Based on these requirements, we decided to measure PPT at three sites: the most inflamed joint, three designated FM point, and two predesignated joints in hands (whether it is inflamed or not). We have studied the change in PPT at different locations and its correlation to core set measures and DAS.
Patients and methods
The prospective observational study was conducted on 20 newly diagnosed RA patients presented to our rheumatology outpatient department between July 2010 and January 2011.The study participants were those who fulfilled ACR 2010 criteria and gave consent to join the trial. The study, approved by the institutional ethical committee, was limited to female between the age group of 30-50 years. The exclusion criteria considered were the following: subjects on opiate medications one week prior to the enrolment in the study, those having overlapping FM, those who couldn’t comprehend the process and give consent, and patients with local dermatological or other conditions that can hinder the PPT measurement.
Patients were assessed for tender joint count, swollen joint count, visual analogue scale (pain/general health), ESR, and CRP during the three consecutive follow-up visits with an interval of six weeks. The time interval between these consecutive visits was six weeks. Tender and swollen joint counts were assessed by an independent joint assessor blinded to PPT results. At each visit, PPT was measured using digital algometer in three pairs of joint areas (third metacarpophalangeals (MCP), wrists, and the most inflamed joint) and three FM tender point sites (suboccipital, mid-trapezius and buttocks). The data on disease duration and medication use were obtained using patient self-report. DMARDs and NSAIDs were administered as prescribed by the rheumatologist.
Evaluation of pressure pain threshold
PPT was measured using digital algometer (Wagner Instruments, FPX 25/220 Algometer) consisting of a soft-grip handle and piston with a pressure-sensitive strain gauge transducer with 1 cm2 probe tip. The participants were allowed to relax in a quiet room and the procedure of PPT was explained to them prior to its initiation. Trial run was performed at sites not considered for evaluation to familiarize the method. The piston of the sensor unit will move inward on applying the pressure and the amount of pressure applied will be shown on the numerical LCD display in kg/cm2. The subject was instructed to give an indication at the onset of pain with the verbal cue “yes”. The reading shown on the LCD meter at that moment was recorded as the pain threshold of the patient. All measures were done on joints of both the sides, except for the most inflamed ones. The pressure was increased at a rate of 1 kg/s to the maximum of 10 kg/cm2. PPT was done in precise sites on each joint. These sites include midpoint as defined in MCP joints, wrist joints (between styloid process of ulna and radius), knee joints (1 cm medial and below the medial border of patella on joint line). PPT was repeated every 20 sec in the same joints and the mean of these measures was calculated. The joint was gently moved (e.g. flexion and extension of proximal interphalangeal joints) before PPT was repeated. The intervals between each algometer measurement were long enough to prohibit temporal summation (TS). Temporal summation occurs when a series of nerve impulses arrives at a synapse, thereby reducing the duration of the impulses less than the postsynaptic potential.12
Statistical analysis
The analysis of the data was done by the calculation of Pearson correlation coefficient, Deming regression, and correlation of change (Δ). The following criteria were adopted for interpreting the magnitude of the correlation: <0.1 trivial, 0.1-0.3 small, 0.3-0.5 moderate, 0.5-0.7 high, 0.7-0.9 very high, 0.9-1.0 near perfect, and 1 as perfect correlation. Mean pain threshold between the first and second trial was calculated for all analyses of the association. The distributions of all the variables were examined. Means and standard deviations (SDs) were calculated for normally distributed variables. Minimum, median, and maximum values were reported for variables that were not normally distributed. Arithmetic means of all areas (average PPT) and for three FM tender points (average PPT of FM points) were computed.
Results
Twenty newly diagnosed female rheumatoid arthritis patients were recruited for the study and all the participants received DMARD treatment during the follow-up. Median disease duration noted was 18 months (Table 1). NSAIDs and steroids were administered to 80% and 35% of the subjects respectively.
Table 1: Patients’ demographic profile
|
Variables |
Values |
|
Age range (yrs) |
30-50 |
|
Median disease duration in months (min, max) |
18 (2, 60) |
|
Rheumatoid factor positive N (%) |
16 (80%) |
|
Anti-CCP positive* |
Nil |
|
Median tender joint count (min, max) |
8 (2, 28) |
|
Median swollen joint count (min, max) |
4 (0, 16) |
|
Median CRP in mg/dl (min, max) |
12.5 (7, 26) |
|
Median ESR in mm/1st hour (min, max) |
68 (22, 114) |
|
Mean DAS28-CRP (SD) |
4.0 (1.02) |
|
Mean DAS28-ESR (SD) |
4.86 ( 1.30) |
|
Median (VAS- GH) (min, max) |
45 (30, 90) |
|
DMARD use N (%) |
20 (100%) |
|
NSAID use N (%) |
16 (80%) |
|
Corticosteroid use N (%) |
7 (35%) |
* Anti-CCP done only in RF negative patient (n = 4) were negative.
1. Pearson correlation coefficient analysis
Average PPT and PPT of the most inflamed joint had moderate to large correlation with DAS-ESR and DAS-CRP, whereas the correlation was small to trivial with ESR and CRP, when they were considered as independent variables (Table 2). Similar results were seen for FM tender point sites.
Table 2: Pearson correlation of PPT with clinical variables recorded during the three follow-up visits
|
Clinical
variables |
Average
PPT |
Average
PPT of FM points |
PPT of
most inflamed joint |
|||||||||||||||
|
Visit 1 |
Visit 2 |
Visit 3 |
Visit 1 |
Visit 2 |
Visit 3 |
Visit 1 |
Visit 2 |
Visit 3 |
||||||||||
|
r |
P |
r |
P |
r |
P |
r |
P |
r |
P |
r |
P |
r |
P |
r |
P |
r |
P |
|
|
DAS-ESR |
-0.766 |
0.001 |
-0.583 |
0.007 |
-0.644 |
0.002 |
-0.741 |
0.000 |
-0.560 |
0.010 |
-0.548 |
0.012 |
-0.575 |
0.008 |
-0.406 |
0.076 |
-0.638 |
0.002 |
|
DAS-CRP |
-0.755 |
0.005 |
-0.592 |
0.042 |
-0.820 |
0.002 |
-0.638 |
0.026 |
-0.554 |
0.062 |
-0.811 |
0.002 |
-0.824 |
0.001 |
-0.468 |
0.125 |
-0.787 |
0.004 |
|
ESR |
-0.036 |
0.881 |
-0.049 |
0.838 |
-0.183 |
0.441 |
-0.211 |
0.371 |
-0.117 |
0.623 |
-0.156 |
0.513 |
0.308 |
0.186 |
-0.021 |
0.929 |
-0.185 |
0.435 |
|
CRP |
-0.192 |
0.550 |
-0.229 |
0.475 |
-0.411 |
0.035 |
-0.204 |
0.525 |
-0.208 |
0.517 |
-0.461 |
0.019 |
-0.315 |
0.319 |
-0.323 |
0.306 |
-0.482 |
0.047 |
2. Deming regression analysis
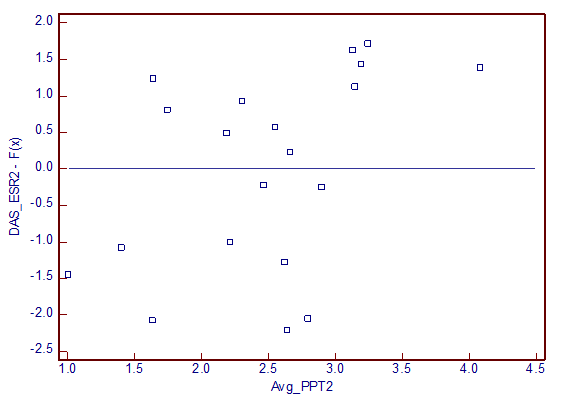
Deming regression analysis (Fig 1 and 2) showed correlation between average PPT and DAS-ESR. Average PPT and average PPT of FM points also correlated with DAS-CRP of all the three visits. Deming regression analysis showed poor correlation of PPT measure with ESR and CRP, when they were considered independently.
Fig 1: Deming regression for predicting DAS-ESR by average PPT recorded on the second follow-up visit
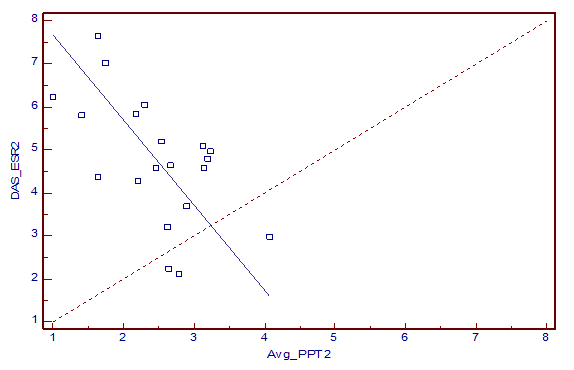
Fig 2: Residual distribution between DAS-ESR and average PPT noted on second follow-up visit
3. Correlation of change in parameters (Δ parameters)
Average PPT vs. DAS-ESR and average PPT vs. DAS-CRP showed moderate to large correlation of change (Table 3). Similar correlation of change was noted for average PPT of FM points. It is interesting to note that even PPT at FM sites showed good correlation with DAS. The correlation of average PPT with DAS-CRP score was found to be better than that of the PPT of the most inflamed joint.
Table 3: Comparison of change in PPT vs DAS and other RA measures (Δ) noted during the three follow-up visits
|
Comparison of |
Δ (visit 2 – visit 1) |
Δ (visit 3 – visit 2) |
|||
|
Changes in PPT |
RA measures |
r value |
P value |
r value |
P value |
|
Δ avg. PPT |
Δ DAS-ESR |
-0.416 |
0.068 |
-0.572 |
0.008* |
|
Δ DAS-CRP |
-0.589 |
0.044* |
-0.606 |
0.048* |
|
|
Δ ESR |
-0.116 |
0.626 |
-0.322 |
0.166 |
|
|
Δ CRP |
-0.027 |
0.910 |
0.148 |
0.533 |
|
|
Δ PPT of most inflamed points
|
Δ DAS-ESR |
-0.281 |
0.231 |
-0.771 |
0.001* |
|
Δ DAS- CRP |
-0.391 |
0.088 |
-0.188 |
0.427 |
|
|
Δ ESR |
-0.111 |
0.642 |
-0.444 |
0.050* |
|
|
Δ CRP |
-0.254 |
0.280 |
-0.037 |
0.877 |
|
|
Δ avg. PPT of FM points |
Δ DAS-ESR |
-0.319 |
0.071 |
-0.354 |
0.025* |
|
Δ DAS-CRP |
-0.339 |
0.044* |
0.066 |
0.075 |
|
|
Δ ESR |
-0.074 |
0.759 |
-0.075 |
0.754 |
|
|
Δ CRP |
-0.025 |
0.917 |
0.402 |
0.079 |
|
* Significance P <0.05
Discussion
Present study demonstrated that the changes in PPT in RA patients correlated significantly with the DAS score. The average PPT also correlated better with the DAS score compared to the measurement of a single, most inflamed joint. Similar correlation was seen for average PPT of FM points. However, the correlation was trivial and small for PPT with inflammatory markers such as ESR and CRP.
Pain measurement is a key component of RA assessment. Increase in pain indicates elevated inflammation in early RA. Whereas in chronic RA, the cause for pain is multifactorial, comprising of both inflammatory and non-inflammatory components (central pain processing mechanisms). Studies have demonstrated that substantiating the correlation of pain sensitivity with change in inflammatory load may aid in monitoring disease activity through the pain sensitivity measurement.5, 7, 8 However, in the present study, the changes in PPT did not correlate significantly with the two inflammatory parameters, ESR and CRP. Number of patients in the current study was not sufficient to conclude this finding. Former studies have also shown such inconsistent relationship between PPT and inflammatory parameters.5 Nevertheless, there is enough literature evidence to indicate that inflammatory cytokines like TNF-alpha and IL6 influence the pain perception at the periphery and centre.6, 10 The present study has focused on the changes in PPT and its correlation to corresponding changes in DAS 28 score (Δ DAS 28) rather than on the absolute value of PPT. Our previously published study (Vijayadas et al., 2013) has clearly indicated a significant reduction in PPT in patients suffering from active RA compared to normal healthy control.13 Additionally, we noted that the change in PPT was not restricted to inflamed joints, but it was observed in the non-inflamed joint areas including designated tender points of FM.
We have considered three different combinations of sites for evaluation, since the change in PPT is systemic and it varies in different parts of the body. Previous studies have highlighted the significance of PPT measurement at different locations to generate a comprehensive assessment of pain sensitivity at joint and non-joint sites.14 Hence, in the present study, we chose three different combinations of sites for PPT measurement to identify the minimum representative sites, which can correlate best with DAS measure. Although measuring PPT of all the joints may be ideal, measuring the same joint twice within an interval of 5-10 sec is cumbersome and time consuming.
We found that the average PPT measures over third MCP, wrist, and the most affected joint in the first visit had significant correlation with both DAS-ESR and DAS-CRP. The correlation was negative, as the DAS score decreased with increase in PPT. Although, the measures of PPT of the most inflamed joint showed correlation in the third visit, the relationship was inconsistent. This can be explained by the frequently encountered clinical situation where the inflamed joint may remain the same during follow-up and the PPT measured for the joint may not change. Whereas the inflammation might be reduced in other joints, thereby reducing the tender and swollen joint counts and decreasing the DAS score. Hence in such patients DAS score may appear reduced during follow-up, but PPT at inflamed joint may not vary as expected. It is interesting to note that PPT, even at non-joint areas (FM points), correlated well with DAS28-ESR (r = -0.62, P = 0.01) and DAS28-CRP (r = -0.67, P = 0.03), suggesting the influence of systemic inflammation on central pain mechanism. The correlation was statistically significant. Further evaluation of the three joint combinations such as MCP, wrist, and the most inflamed joint was also significant to substantiate the study results.
Studies have validated the accuracy of measuring PPT, especially with single observer, in different painful conditions. PPT measurement has also shown high inter- and intra- observer repeatability without significant differences.15 The study is not affected by the fact that PPT varies in each individual, since we have measured the change in absolute values of PPT in the same person and its relationship to disease activity.
The distinct advantage of PPT is that the measurement is not influenced by use of NSAIDs, whereas both VAS and number of tender joints are altered by the NSAID use.16 Therefore, the use of PPT can be considered superior to tender joint and VAS. In inflammatory arthritis, TNF-alpha and IL6 can induce sensitivity to pain at joint and non-joint areas. Sometimes the number of tender joints may not decrease sufficiently in patients undergoing treatment with DMARDs and with well-controlled inflammation. Hence there may not be a significant change in DAS28. In spite of the reduced inflammatory load, the presence of persisting tender joints and higher DAS, may falsely suggest higher disease activity and this can be fairly measured by changes in PPT.17 In our previous study, we have observed a gap between VAS representation of pain and lower PPT when comparing healthy normal controls with FM and RA patients.13 The measured gap between PPT and VAS representation of pain could help in determining the cause for pain (whether pain is due to inflammation or central perception mechanism). This may assist physicians in deciding treatment strategies, such as the use of NSAIDs or duloxetine and gabapentin, depending on the pain pathway.17
Present study has certain limitations. We analyzed a relatively small number of participants, (n=20). The power of the study was poor owing to the possibility of a wider range of variable PPT and inflammatory parameters. Strictly controlled environment was not achieved with respect to NSAID use. A majority of the participants (80%) was receiving oral analgesics, which could have altered algometer pain thresholds changes. Medications influence PPT measurements and DAS score. The confounding effect is minimal in the present study, since we have focused on the changes in PPT and its correlation to corresponding changes in DAS 28 score (Δ DAS28) rather than on the absolute value of PPT, which is affected by medications. Opiate medications strongly affect PPT through their sustained analgesic effects, so patients on opiate medications were excluded from the study. A recent study of OA rodent model reported that treatment with NSAIDs only showed transient analgesic effects, whereas the effects of centrally-acting analgesics, including amitriptyline and gabapentin, were more sustained.12 Although the present study is preliminary and need further evaluation with larger number of patients in more controlled environment, it substantiates the fact that measuring PPT and its changes in RA patients may aid in measuring disease activity in conjunction with DAS.
In addition, the present study indicating the relationship between disease activity and pain threshold at joint and non-joint sites provides novel insights regarding pain mechanisms. Peripheral mechanisms, such as peripheral sensitization, are characterized by local areas of hyperalgesia/allodynia in response to experimental induction of inflammation. Whereas, the widespread effects of central mechanisms involve both joint and non-joint sites.18 Previous studies in RA patients have demonstrated enhanced reactivity to pain and low pain threshold at joints and non-joints sites than healthy controls due to the defect in central pain processing like central augmentation and sensitization.10, 18, 19 In contrast to the previous studies, the present one has focused on changes in PPT with treatment and demonstrated that the changes correspond to Δ DAS28. Measuring PPT, in contrast to VAS in RA, especially in the presence of FM, has unique advantage in circumventing high pain levels that can alter VAS-GH and tender joint count.
The study was conducted only in female patients to avoid sex as a confounding variable and considering the fact that RA is more common among women. Moreover, It is well recognized that there is gender difference in pain perception and PPT changes are easily appreciated by female patient.20, 21, 19 Even though there is a significant variation in absolute value of PPT between men and women, the current study has focused on the change of absolute values in PPT and its relationship to disease activity in the same individual. Hence, these observations can be extrapolated to male population also. However, further research to substantiate these findings in male is required.
It could be concluded that alteration in RA disease activity is reflected by the changes in pain sensitivity, which can be objectively measured with algometer. Change in PPT has fair correlation with DAS, suggesting its application along with DAS for documenting disease activity and management of RA. DAS score is relatively complex for immediate determination in the daily clinical setting and requires extensive calculation. However, measuring PPT is an intuitive and easy way to assess disease activity in daily clinical practice. Further evaluation with other inflammatory markers like IL-6 and TNF-alpha is required to establish correlations with PPT.
Competing interests
The authors declare that they have no competing interests.
Disclosure of funding
The research work is sponsored by the institutions in which the authors are working
References
1. Sokka T. Assessment of pain in patients with rheumatic diseases. Best Pract Res Clin Rheumatol. 2003;17:427-49.
2. Prevoo M L, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44-8.
3. Pincus T, Yazici Y, Sokka T. Quantitative measures of rheumatic diseases for clinical research versus standard clinical care: differences, advantages and limitations. Best Pract Res Clin Rheumatol. 2007;21:601-28.
4. van Gestel AM, Prevoo ML, van't Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996;39: 34-40.
5. Lee YC, Chibnik LB, Lu B, Wasan AD, Edwdards RR, Fossel AH, et al. The relationship between disease activity, sleep, psychiatric distress and pain sensitivity in rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 2009;11:R160.
6. De Jongh RF, Vissers KC, Meert TF, Booij LH, De Deyne CS, Heylen RJ. The role of interleukin-6 in nociception and pain. Anesth Analg. 2003;96:1096-1103.
7. Wieseler-Frank J, Maier SF, Watkins LR. Central proinflammatory cytokines and pain enhancement. Neurosignals. 2005;14:166-74.
8. Alstergren P, Fredriksson L, Kopp S. Temporomandibular joint pressure pain threshold is systemically modulated in rheumatoid arthritis. J Orofac Pain. 2008;22:231-38.
9. Chesterton LS, Sim J, Wright CC, Foster NE, Chesterton LS, Barlas P, et al. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. Clin J Pain. 2007;23:760-66.
10. Robert RE, Ajay DW, Clifton OB, Joan B, Jennifer AH, Michael TS, et al. Enhanced reactivity to pain in patients with rheumatoid arthritis. Arthritis Res Ther. 2009; 11:R61.
11. Chandrashekra S, Sachin S. Measures in rheumatoid arthritis: are we measuring too many parameters. IJRD. 2012;15:239-48.
12. Ivanavicius SP, Ball AD, Heapy CG, Westwood FR, Murray F, Read SJ. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: increased expression of ATF-3 and pharmacological characterisation. Pain. 2007;128:272-82.
13. Vijayadas R, Arun Kaushik MS, Roopakala, Chandrashekra S. Effect of pressure pain threshold in fibromyalagia syndrome and rheumatoid arthritis patients. Al Ameen J Med Sci 2013;6:112-19.
14. Edwards RR, Sarlani E, Wesselmann U, Fillingim RB. Quantitative assessment of experimental pain perception: multiple domains of clinical relevance. Pain. 2005;114:315-19.
15. Persson AL, Brogårdh C, Sjölund BH. Tender or not tender: test-retest repeatability of pressure pain thresholds in the trapezius and deltoid muscles of healthy women. J Rehabil. Med. 2004;36:17-27.
16. Christidis N, Kopp S, Ernberg M. The effect on mechanical pain threshold over human muscles by oral administration of granisetron and diclofenac-sodium. Pain. 2005;113:265-70.
17. Ton E, Bakker MF, Verstappen SM, Ter Borg EJ, van Albada-Kuipers IA, Schenk Y, et al. Look beyond the disease activity score of 28 joints (DAS28): tender points influence the DAS28 in patients with rheumatoid arthritis. J Rheumatol. 2012;39:22-7.
18. Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther. 2011;13:211.
19. Gerecz-Simon EM, Tunks ER, Heale JA, Kean WF, Buchanan WW. Measurement of pain threshold in patients with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, and healthy controls. Clin Rheumatol. 1989;8:467-74.
20. Pieh C, Altmeppen J, Neumeier S, Loew T, Angerer M. Gender differences in outcomes of a multimodal pain management program. Pain. 2012;153:197–202.
21. Racine M, Tousignant-Laflamma Y, Kloda LA, Dion D, Dupuis G. A systematic literature review of 10 years of research on sex/gender and experimental pain perception— part 1: are there really differences between women and men?. Pain. 2012;153:602–18.