Case Studies
Presentation of Takayasu’s arteritis in a patient with intervertebral disc prolapse
Jayashankar CA1*, Somasekar DS2, Prachi Kala3, Bhanu Prakash4, Varsha Reddy K5, Srikar Inuganti6
Author Affiliations
1Associate Professor, Department of General Medicine, Vydehi Institute of Medical Sciences and Research Centre, Bangalore, India
2Professor and Head, Department of General Medicine, Vydehi Institute of Medical Sciences and Research Centre
3Professor, Department of Radiology, Vydehi Institute of Medical Sciences and Research Centre
4Professor, Department of Dermatology, Vydehi Institute of Medical Sciences and Research Centre
5Post graduate, Department of General Medicine, Vydehi Institute of Medical Sciences and Research Centre
6Post graduate, Department of Radiology, Vydehi Institute of Medical Sciences and Research Centre
* Correspondence: Dr. Jayashankar CA
IJRCI. 2014;2(1):CS1
Received: 3 January 2014, Accepted: 22 January 2014, Published: 31 January 2014
© IJRCI
Abstract
Takayasu’s arteritis is an idiopathic, chronic inflammatory disease affecting large and medium sized arteries, especially aorta, its major branches, and the pulmonary and coronary arteries. Though the disease incidence is very rare, it may lead to prolonged morbidity and mortality. The clinical presentations are more often due to ischemic features. In certain cases, the presentation may be atypical to consider the diagnosis of Takayasu. We present here the case of a young woman diagnosed with Takayasu’s arteritis who presented with features of intervertebral disc prolapse.
Introduction
Takayasu’s arteritis (TA) is an idiopathic chronic granulomatous inflammatory disease of large and medium sized arteries primarily affecting aorta and its branches.1 The disease is relatively uncommon and majority of the cases are reported from Japan, India, China, Sri Lanka, and Singapore.1
Case report
A 33-year-old female, working as a mason, presented to the hospital with a history of low backache since past 1 year and pain in both the lower limbs since 3 months. Low backache of moderate intensity was insidious in onset, and it progressively radiated to buttocks during each episode. It got worsened on bending forward, coughing, and sneezing. Lower limbs pain, which was felt in the calves, worsened on walking for about half a kilometer and relieved on rest. However, no history of motor weakness and paresthesia of lower limbs was reported. The patient also experienced loss of appetite and loss of weight of approximately 10 kg since last 6 months. There was no history of cough with expectoration, evening rise of temperature, trauma to lower back, hypertension, diabetes mellitus, ischemic heart disease and menstrual abnormalities.
General physical examination revealed that the patient was moderately built and poorly nourished. Both upper and lower limbs pulses were very feeble and the blood pressure was not recordable. A thrill was felt in carotid arteries and no clubbing, cyanosis, lymphadenopathy, pedal edema, and goiter was recorded. On cardiovascular examination, there was short early diastolic murmur of grade 2/6 heard in aortic area with loud first and second heart sounds in all areas. No abnormal findings were reported during respiratory, abdominal and nervous system examinations. Chest X-ray was normal. The significant findings of lab investigations are given in table 1.
Table 1: Significant findings of lab investigations
|
Parameters evaluated |
Values |
|
Hb |
7.6 gm% |
|
Total leucocyte count |
8,910/ µL |
|
Platelet count |
2,20,000/µL |
|
ESR |
70 mm/hr |
|
C-reactive protein |
7.23 mg/dl |
|
RA factor |
Negative |
|
ANA, p-ANCA and c-ANCA |
Negative |
|
Albumin |
2.5g/dl |
|
Globulin |
4.9 g/dl |
Electrocardiogram revealed sinus tachycardia with heart rate of 130/min. 2D echocardiogram showed mild concentric left ventricular hypertrophy, moderate left ventricular global hypokinesia, grade III left ventricular diastolic dysfunction, and ejection fraction of 52%. Doppler of both carotid arteries revealed homogeneous circumferential thickening of affected vessels with flow velocity elevations beyond stenotic segments. Ultrasound examination of abdomen and pelvis revealed small right kidney with right renal arterial stenosis.
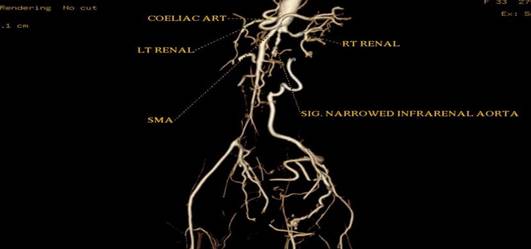
CT aortic angiography demonstrated concentric arterial wall thickening of nearly the whole aorta, its branches, and the pulmonary trunk (Figure 1). Severe stenosis was noted involving the origins of right brachiocephalic artery, left subclavian and left common carotid arteries (Figure 2). The pulmonary arteries beyond bifurcation were normal with no evidence of wall thickening. Wall calcification was present in the suprarenal aorta and superior mesenteric artery. Significant luminal narrowing of abdominal aorta was noted in the infrarenal segment with total occlusion of aorta up to bifurcation. Reformation of the external and internal iliac arteries from the collaterals arising from mesenteric arteries was noted. The infrarenal aortic branches were not visualized (Figure 3). Aneurysmal dilatation of the supra renal abdominal aorta and left renal artery was noted. No inflammatory infiltrates, granulomas or giant cells were seen in right superficial temporal artery biopsy.
Figure 1: CT angiogram showing circumferential wall thickening of
descending aorta
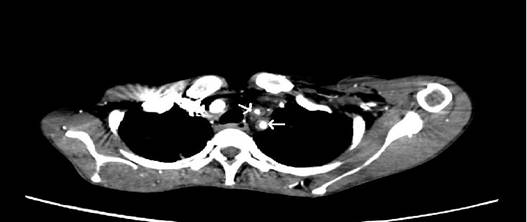
Figure 2: CT angiogram showing narrowed origins of left common carotid
and left subclavian artery with wall thickening
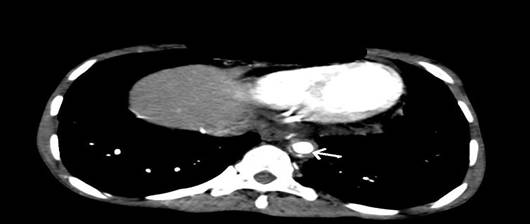
Figure 3: Volume-rendered image of CT angiography indicating segmental
occlusion of infrarenal aorta with a narrowed right renal artery
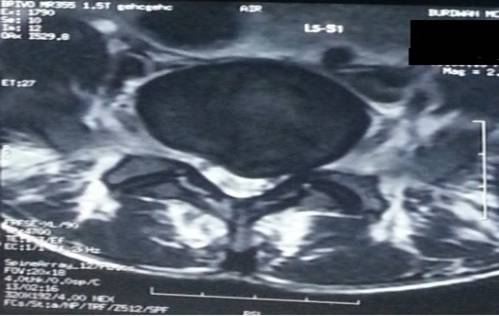
MRI scan of LS spine (Figures 4 and 5) revealed disc desiccation with left paracentral disc bulge at L5- S1 level, causing left foraminal stenosis and left exiting nerve root compression.
Based on the clinical evaluation, the diagnosis was confirmed as TA with compressive radiculopathy secondary to L5-S1 disc prolapse and moderate normocytic normochromic anemia. The patient refused any surgical intervention and she was treated orally with methotrexate 10 mg/week, prednisolone 20 mg twice daily, and NSAIDS. A good symptomatic relief was observed two weeks after the initiation of the treatment. She was discharged and advised to do follow-up once in 3 months to identify any systemic manifestations.
Figure 4: Sagittal T2 weighted MRI image showing disc bulge at L5-S1 level
indenting the thecal sac
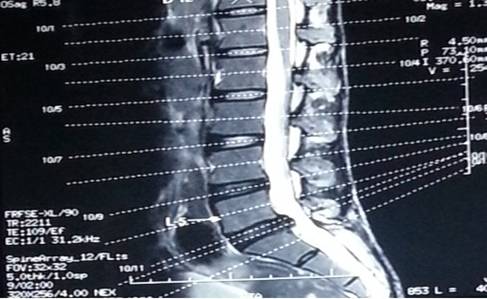
Figure 5: MRI of LS spine showing left paracentral disc bulge at L5- S1 level
causing left foraminal stenosis and left exiting nerve root compression
Discussion
The present case, fulfilled most of the American College of Rheumatology criteria for TA including claudication pain in both lower limbs, decreased pulses, angiographic evidence of narrowing or occlusion of aorta and its branches.2 The radicular pain noted in the present case was due to nerve root compression developed secondary to disc prolapse. The patient was evaluated further for reduced or absent pulse, in addition to root compression. The common clinical presentation of TA are a) reduced /absent pulses in 84-96% of cases b) bruit in 80-94% c) hypertension in 33-83% d) renal artery stenosis in 28-75% e) aortic regurgitation in 20-24% and f) pulmonary artery involvement in 14-100%.3
The exact etiopathogenesis of TA is unknown and its causal relationship with any of the aforementioned abnormalities has not been well established.4 It has been proposed that an increased expression of heat shock protein 60 in combination with a special population of T cells leads to cytotoxic reaction in TA patients and eventual damage of the aortic endothelium.5
The disease often begins as an inflammation of tunica adventitia, subsequently involving tunica media and tunica intima leading to thickening, fibrosis, segmental stenosis, aneurysm and thrombus formation.6 It progresses in a triphasic pattern. Phase I is the systemic or pre-pulseless period characterized by constitutional symptoms and phase II is the vasculitic stage where constitutional symptoms are accompanied by features of vascular involvement. Phase III is the late, fibrotic, occlusive, phase, wherein characteristic features of TA (pulseless disease) related to arterial stenosis or occlusion appear.6 Various criteria proposed for diagnosis of TA include American College of Rheumatology criteria, Ishikawa’s criteria, and modified diagnostic criteria for TA by Sharma et al. 2, 7, 8
In the current case study, ANA was negative VDRL was nonreactive, and the characteristic features of sarcoidosis like skin lesions, hilar adenopathy on chest X-ray, and Bell’s palsy were absent. The presence of giant cell arteritis was ruled out, since the patient was young and the result of superficial temporal artery biopsy was normal. As per the literature evidence, around 25% of the cases of TA were diagnosed at > 40 years of age.9 The pickup rate of TA is high in patients with suspected diagnosis. The current case illustrates such an example. Though clinical presentation was backache with radicular pain, unexplainable symptoms led to the diagnosis.10
Conclusion
TA should be considered in women with constitutional features, hypertension, absence of pulse or low volume pulse, and carotid artery bruit. Early diagnosis and institution of appropriate treatment is crucial to prevent further progression and complications like aortic regurgitations and cerebrovascular accidents.
Competing interests
The authors declare that they have no competing interests.
References
1. Jain S, Kumari S, Ganguly NK, Sharma BK. Current status of Takayasu arteritis in India. Int J Cardiol 1996;54 suppl:111-6.
2. Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990 Aug;33(8):1129–34.
3. Johnston SL, Lock RJ, Gompels MM. Takayasu arteritis: a review. J Clin Pathol 2002;55:481-6.
4. Bali HK, Bali SK, Jain AK .Takayasu arteritis. J K Science. 1999 April- June; Vol I (2): 3-10
5. Chauhan SK, Singh M, Nityanand S. Reactivity of gamma/delta T cells to human 60-kd heat-shock protein and their cytotoxicity to aortic endothelial cells in Takayasu arteritis. Arthritis Rheum 2007; 56:2798- 802.
6. Vaideeswar P, Jaya R. Pathology of Takayasu arteritis: A brief review. Annals of Pediatric Cardiology 2013; 6(1):52-8.
7. Ishikawa K. Diagnostic approach and proposed criterion for the clinical diagnosis of Takasyasu arteriopathy. J Am Coll Cardiol1988; 12: 964-972.
8. Sharma BK. Jain S, Suri S. Numano F. Diagnostic criterion for Takayasu's arteritis. Int J Cardiol 1996: 54 (Suppl I):S 127-133.
9. Richards BL, March L, Gabriel SE. Epidemiology of large vessel vasculitides. Best Pract Res Clin Rheumatol 2010; 24:871
10. White C. Clinical practice. Intermittent claudication. N Engl J Med. 2007 Mar 22;356(12):1241-50.