Original
Articles
Diagnosis
of circulatory antibodies to Chlamydia
trachomatis among asymptomatic undifferentiated spondyloarthropathy
patients in India
Praveen
Kumar1, Geetika Khanna2, Sumit Batra3, Vinod
Kumar Sharma4, Sangita Rastogi5*
Author Affiliations
1 Senior Research Fellow, Microbiology
laboratory, National Institute of Pathology (ICMR), Safdarjung hospital campus,
New Delhi, India
2 Professor in Pathology, Central Institute of
Orthopedics (CIO), Vardhaman Mahavir Medical College (VMMC) & Safdarjung
hospital, New Delhi, India
3 Specialist Orthopaedic Surgeon, Central
Institute of Orthopedics (CIO),Safdarjung hospital, New Delhi, India
4 Director Professor & HOD, Central
Institute of Orthopedics (CIO), Safdarjung hospital, New Delhi, India
5Scientist ‘E’ & Deputy Director, Microbiology
laboratory, National Institute of Pathology (ICMR), Safdarjung hospital campus,
New Delhi, India
* Correspondence: Dr. Sangita Rastogi
rastogi_sangita@rediffmail.com
IJRCI.
2013;1(S1):SO1
Received: 26 July 2013, Accepted: 3 October 2013,
Published: 5 November 2013
© IJRCI
Abstract
Background
C. trachomatis infection has been reported in patients with undifferentiated spondyloarthropathy (uSpA). However, it is underdiagnosed globally, including India.
Aim
The aim of the study was to determine circulatory anti-Chlamydia trachomatis antibodies in uSpA
patients.
Materials and methods
Twenty two uSpA patients were included in the study following the
European Spondylarthropathy Study Group (ESSG) criteria and 12 age- and sex-
matched osteoarthritis patients were also included for comparison. From each
patient, 5 ml of non-heparinized intravenous blood was collected for analysis.
Anti-C. trachomatis IgG and IgA
antibodies were detected in serum using commercially available ELISA kits and the
data was statistically evaluated.
Results
During the study, 18.1% (4/ 22) uSpA patients were found positive for
anti-C. trachomatis IgA antibodies,
while 4.5% (1/ 22) patients were positive for anti-C.trachomatis IgG antibodies in serum. None of the control patients
was positive for these antibodies.
Conclusion
C.
trachomatis may be an unrecognized cause for uSpA even among Indian
patients. Further studies involving larger study population are significant to
substantiate the findings.
Introduction
Reactive arthritis (ReA), a non-canonical septic, inflammatory arthritis commonly associated with Chlamydia trachomatis infection, represents a significant health burden, yet it is poorly understood and underestimated.1 Another important entity, viz. undifferentiated spondyloarthropathy (uSpA) encompasses a group of arthritic patients who do not get categorized into any defined spondyloarthropathy, viz.: ankylosing spondylitis, psoriatic arthritis, ReA or arthritis associated with chronic inflammatory bowel disease and are considered to be ‘forme fruste’ of ReA.2, 3 Patients with uSpA may or may not present with typical clinical symptoms like synovitis in joints. Broadly, the only difference with ReA is the absence of urogenital symptoms. C. trachomatis causes chronic infection and it becomes persistent and untreatable in joint and this asymptomatic form makes this pathogen an unrecognized cause of ReA.4 Numerous published studies on ReA/uSpA from our country have focused on enteric infections.4, 5 As the prevalence of genital C. trachomatis is found to be high in India, there is a definite need to investigate the presence of C. trachomatis in ReA/uSpA patients.6, 7 Recently, our group reported identifying intra-articular C. trachomatis infection by both PCR and immunofluorescence in genitourinary ReA as well as in uSpA patients.8, 9 Since screening is necessary, the aim of the study was to screen asymptomatic uSpA patients for the presence of serum anti-C. trachomatis IgG/IgA antibodies.
Materials and Methods
With the permission of hospital ethics committee, 34 age-matched uSpA (mean age- 30.7 years) and OA patients (mean age- 34.6 years) attending Department of Orthopedics at Safdarjung Hospital, New Delhi were enrolled. Among these, 22 were uSpA patients, while the control group of OA comprised of 12 patients. A detailed clinical history including any previous infection, treatment or any other extra-articular symptoms was recorded. uSpA patients were recruited following ESSG criteria.10 Clinical and radiological diagnosis was done for OA patients. Informed written consent was obtained from each patient. Patients with enteric/ tubercular/ viral infection or any other defined arthropathies were excluded.
Intravenous blood of 5.0 ml was drawn under aseptic condition and the serum was separated and stored at -20°C for conducting various assays. The detection of circulatory IgG and IgA antibodies to C. trachomatis was done by ELISA in the sera of arthritic patients using commercially available kits (IBL International, Germany and Savyon Diagnostics, Israel, respectively), following the manufacturer’s guidelines. Briefly, 10 µl of diluted sera of patients and controls were pipetted into wells, incubated (for binding of C. trachomatis-specific IgG/IgA antibodies) and washed. This was followed by the addition of horseradish peroxidase-conjugated anti-human IgG/IgA antibodies, incubation, and washing. 3,3',5,5'-tetramethylbenzidine (TMB) substrate was added for color development and the reaction was stopped by sulphuric acid and the plate was read at an absorbance of 450 nm. Anti-C. trachomatis IgG antibodies were calculated in Units (U) according to the formula: patient (mean) absorbance value x 10/ cut-off, where cut-off = 10 U. Patients with >11 U were considered positive, <9 U were negative and between 9-11 U were in the grey zone. The cut-off value (COV) was calculated for C. trachomatis-specific IgA antibodies according to the formula: COV= NC x 2, where NC= average absorbance at 450 nm of the negative control run in duplicate, while the cut-off index (COI) was calculated according to the formula: COI= absorbance of serum sample at 450 nm divided by COV. Patients with COI >1.1 were considered as positive, COI <1.0 as negative, while those with COI between 1 - 1.1 were borderline cases.
Non-parametric Mann-Whitney test was performed for comparing different groups. Fisher exact test was performed for different variables. Statistical analysis was performed with GraphPad Prism software version 5.0 (GraphPad Software, Inc., San Diego, California, USA).
Results
Age-matched 34 arthritic patients (22 uSpA, 12 OA) were subjected to C. trachomatis screening by commercially available antibody detection kit in blood. The mean age of uSpA patients belonging to the study group was 30.7 years. M:F ratio was 15:7 and the mean disease duration was 18.6 months. Oligoarthritis was seen in 68% (15) patients and synovitis of major joints (knee/ ankle) in 45.5% (10) patients. Majority (95.4%) of the patients (n=21) in the study group had low backache and enthesitis was reported in 9% (n=2) of the subjects. Serum C-reactive protein was found to be 29.6 + 16.1 µg/ml (mean + S.D.) in uSpA patients, while it was 4.6 + 1.2 µg/ml (mean + S.D.) in patients with OA. Overall, 18.1% (4/ 22) uSpA patients found positive for anti-C. trachomatis antibodies in the serum. All positive patients had an oligoarthritic pattern in joints and had high level of C-reactive protein (> 57 µg/ml) (Table 1). 18.1% (4/ 22) patients were positive for anti-C. trachomatis IgA antibodies, while 4.5% (1/ 22) patients were found positive for anti-C. trachomatis IgG antibodies in serum. One patient was found to be positive for both antibodies. Comparison of IgA cut-off index value is shown in Fig. 1.
Table 1: Clinical
characteristics of Chlamydia trachomatis-positive
undifferentiated spondyloarthropathy patients
|
Age(yrs)/
Sex |
Disease Duration (months) |
Type
of arthritis |
IgG Abs
to Ct |
IgA Abs to
Ct |
CRP (µg/ml) |
IgG Units |
IgA COI |
|
18/ F |
6 |
O |
+ |
+ |
60 |
12 |
1.7 |
|
21/ M |
6 |
O |
- |
+ |
63 |
- |
1.69 |
|
21/ M |
6 |
O |
- |
+ |
83 |
- |
2.6 |
|
21/ M |
6 |
P |
- |
- |
57 |
- |
1.8 |
F:
female; M: male; yrs: years; abs: antibodies; Ct: chlamydia trachomatis; CRP: C-reactive protein; COI: cut-off index;
O: oligoarthritis; P: polyarthritis
Fig 1: Bar diagram showing
difference in cut-off index value in study and control groups
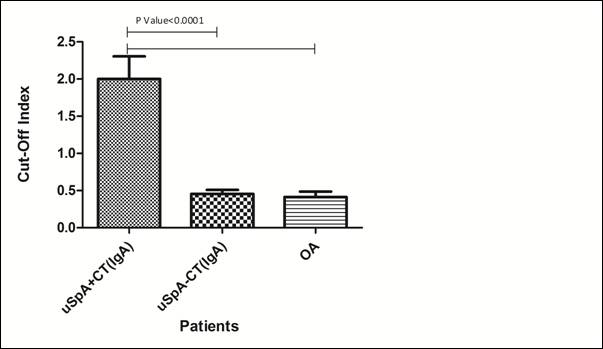
uSpA+CT: undifferentiated spondyloarthropathy patients
with C. trachomatis infection
uSpA-CT: undifferentiated spondyloarthropathy patients
without C. trachomatis infection
OA: Osteoarthritis
‘p’ value <0.05 considered to be significant
Discussion
uSpA is the most common presentation among various spondylarthritides.11 However, the exact prevalence of uSpA is difficult to assess quantitatively as the disease is often unrecognized.3 In this regard, C. trachomatis was found to be the most important pathogen in patients diagnosed with an asymmetrical oligoarthritis and having no preceding history of infection. Although, the serodiagnosis of C. trachomatis is not well appreciated in various diseases, it can serve as an initial non-invasive diagnostic method and can be utilized in uSpA patients without having any symptoms suggestive of chlamydia infections, which may cause persistent infection in later stages. It can also be used in cases where nucleic acids are either undetectable or absent. The specificity and sensitivity for determination of IgG antibodies in joints is 80%. The specificity reaches 90% with the determination of synovial fluid IgA antibodies.12 Diagnosis of C. trachomatis infection in uSpA is difficult even in active or silent phase of infection. During antibiotic treatment or in self-limiting condition/relapsing phase, antibody screening may assist in C. trachomatis detection in the serum as the DNA of this pathogen becomes undetectable by PCR method. Screening for anti-C. trachomatis antibodies may work as a prognostic marker and can be performed at intermittent periods for diagnosis of antibodies to circulatory C. trachomatis antigens like lipopolysaccharide (major outer membrane protein) in serum. If any patient is found positive for C. trachomatis antibodies in serum, further molecular diagnostic tests can be performed for confirmation. This is particularly important for the male patients who are at higher risk of developing ReA/uSpA in comparison to female due to its asymptomatic nature in male. Our observations on detection of C. trachomatis IgG/IgA antibodies in asymptomatic uSpA patients were further strengthened by the fact that all positive subjects had higher serum C-reactive protein levels, thereby indicating bacterial infection. These patients were also assessed for extra-articular infection. However, none had any such manifestations.
Current study showed that uSpA patients who were asymptomatic for urogenital infection were also at risk of developing C. trachomatis-induced ReA. It is significant to understand the magnitude of disease burden in larger number of asymptomatic uSpA patients in our country to prevent chlamydial infection at an earlier stage by employing improved management strategies.
However, in the present study, the results obtained only in OA patients were reported. Since OA patients serve as non-inflammatory controls, it would have been more appropriate to enroll inflammatory disorder patients such as those with rheumatoid arthritis also as controls for assessing non-specific antibody production. Another limitation of the study is that serology alone cannot convincingly diagnose chlamydial infection and also cannot replace the sensitivity and specificity associated with molecular diagnostic tests. However, it can serve as an initial non-invasive screening method during an early diagnosis of C. trachomatis infection, but the results obtained will require further confirmation before therapeutic intervention.
Acknowledgements
Praveen
Kumar acknowledges the award of Direct Senior Research Fellowship by Indian
Council of Medical Research, New Delhi (India).
Competing interests
The
authors declare that they have no competing interests.
References
1. Eric
G, Robert DI. Chlamydia-induced ReA: Immune imbalances and persistent
pathogens. Nat Rev Rheumatol. 2012; 8: 55–9.
2. Aggarwal
A, Misra R, Chandrasekhar S, Prasad KN, Dayal R, Ayyagari A. Is
undifferentiated seronegative spondyloarthropathy a forme fruste of reactive
arthritis? Rheumatology 1997; 36: 1001-4.
3. Carter
JD, Gérard HC, Espinoza LR, Ricca LR, Valeriano J, Snelgrove J, Oszust C, Vasey
FB, Hudson AP. Chlamydiae as etiologic agents in chronic undifferentiated
spondylarthritis. Arthritis Rheum. 2009; 60(5): 1311-6.
4. Kathryn
S. Chlamydia: A much underestimated STI. The Lancet Infectious Diseases 2012;
12(7): 517-18.
5. Chandrashekara
S, Aggarwal A, Prasad K N, Misra R. Sporadic reactive arthritis in north India:
Lack of IgA Response. J Indian Rheumatol Assoc. 2004; 12: 134-38.
6. Rastogi
S, Das B, Mittal A. Serum IgM to Chlamydia trachomatis in pregnancy: Its
usefulness for screening. Br J Biomed Sci. 2002; 59 (1): 30-4.
7. Rastogi
S, Das B, Salhan S, Mittal A. Effect of treatment for Chlamydia trachomatis
during pregnancy. Int J Gynecol Obstet. 2003; 80: 129-37.
8. Kumar
P, Bhakuni DS, Mullick G, Kartik S, Rastogi S. Diagnosis of Chlamydia
trachomatis in joint fluid of chronic reactive arthritis and undifferentiated
spondyloarthropathy patients. Indian Journal of Rheumatology 2012; S (7):
S1-S6.
9. Kumar
P, Khanna G, Batra S, Sharma VK, Rastogi S. A pilot study for detection of
intra-articular chromosomal and extra-chromosomal genes of Chlamydia
trachomatis among genitourinary reactive arthritis patients in India.
International Research Journal of Medical Sciences 2013; 1(4): 16-20.
10. Dougados
M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, et al &
European Spondylarthropathy Study Group. The European Spondyloarthropathy Study
Group preliminary criteria for the classification of spondyloarthropathy.
Arthritis Rheum. 1991; 34: 1218-27.
11. Boyer
GS, Templin DW, Bowler A, Lawrence RC, Heyse SP, Everett DF, Cornoni-Huntley
JC. Spondyloarthropathy in the community: Clinical syndromes and disease
manifestations in Alaskan eskimo populations. J Rheumatol. 1999; 26(7):
1537–44.
12. Bas
S, Vischer TL. Chlamydia trachomatis antibody detection and diagnosis of
reactive arthritis. Br J Rheum. 1998; 37: 1054-59.