Case Studies
Lupus erythematosus panniculitis presenting with initial periorbital swelling and positive antiphospholipid
antibodies: A rare association
Jyotsna
Oak1*, Sama Rais2, Shankar Sawant3
Author Affiliations
1 Consultant Rheumatologist and Physician,
Kokilaben Dhirubhai
Ambani Hospital, Mumbai, India
2, 3 Consultant Dermatologist, Kokilaben Dhirubhai Ambani Hospital, Mumbai, India
* Correspondence: Dr. Sama Rais
IJRCI. 2013;1(1):CS6
Received: 10 July 2013, Accepted: 29 August 2013,
Published: 4 October 2013
© IJRCI
Abstract
Lupus erythematosus panniculitis (LEP), an unusual form of chronic cutaneous lupus erythematosus, is characterized by chronic inflammation and fibrosis of subcutaneous tissue. Clinically, it presents as subcutaneous nodules on common locations such as forehead, cheeks, proximal limbs and buttocks. Ulceration of the nodules may occur in certain cases. Very few case studies have reported the occurrence of early solitary periorbital involvement, highlighting the need for a high index of suspicion in such cases. We report here a case having generalized extensive LEP with initial manifestation of a solitary periorbital swelling, autoimmune hemolytic anemia, and associated antiphospholipid antibodies positivity.
Introduction
Lupus erythematosus panniculitis (LEP) is a rare presentation of cutaneous lupus erythematosus seen only in about 2 to 7% of the patients with systemic lupus. It can also occur prior to the manifestation of systemic or discoid lupus erythematosus. The clinical manifestation involves the presence of subcutaneous nodules with adherent overlying skin, which are commonly located on the forehead, cheeks, proximal limbs and buttocks. The association of antiphospholipid antibody with LEP is very rare and only three cases have been reported till date in literature.
Case report
A 34-year-old female presented with complaints of subcutaneous warm tender nodules. They were first noticed over the right periorbital area with subsequent development of such lesions over arms, legs, and buttocks since two months. The nodules increased in size progressively and developed to ulceration (Fig 1 and 2). A sudden increase in the right periorbital swelling within 10 days was observed, causing inability to open the eye and painful eye movements without any diminution of vision (Fig 3).
Fig1: Presence of tender erythematous
subcutaneous nodule just above the right knee
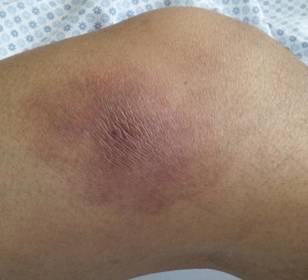
Fig 2: Occurrence
of tender ulcerated subcutaneous nodules over the flexor aspect of the right
upper arm
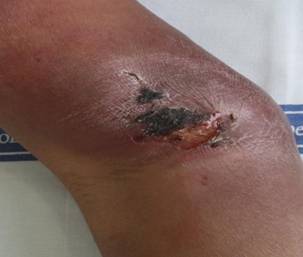
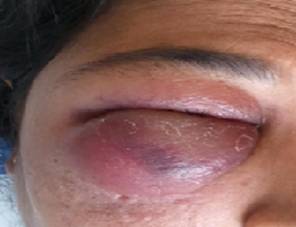
Fig 3: Swelling
and inability to open the right eye
There was no history of trauma or insect bite preceding the lesions. She was experiencing low grade fever and intermittent arthralgia since two months. She denied the occurrence of photosensitivity, malar or discoid rash, myalgia, fatigue, swelling of feet, Raynaud's phenomenon, dyspnea, seizures or psychosis, loss of weight or appetite, cough with expectoration, abdominal pain, diarrhea or constipation. There was no history suggestive of anesthetic hypopigmented patches or tingling numbness. However, a past history of pulmonary tuberculosis was reported around three years ago and the patient was on antitubercular treatment for one year. Around five months back, a therapeutic trial of antitubercular treatment was given for two months at another hospital suspecting the occurrence of subcutaneous nodules as cutaneous tuberculosis. But the treatment was discontinued as no improvement was noticed.
Family history of the patient was negative for tuberculosis, Hansen’s disease, and complement deficiency. Physical examination found that her vital parameters were normal. She had deforming, widespread indurated plaques on the face, upper extremities and buttocks. There was no periungual telangiectasia or subungual splinter hemorrhages. The results of clinical and lab investigations are given in table 1.
Table1: Results of
clinical and lab investigations
|
Investigations |
Results |
|
Hemoglobin |
9.4 g/dl |
|
Total leukocyte count |
3500 /cumm |
|
Lymphocytes 8%, basophils 0%, eosinophils 0%, neutrophils 91% |
|
|
ESR |
80 mm in 1 hour |
|
Urine routine microscopy |
Protein +, rest within normal limits |
|
Serum creatinine |
0.8 mg/dl |
|
X-ray of chest |
Normal |
|
Ultrasonography of abdomen |
Mild splenomegaly, rest normal |
|
Direct Coomb’s test |
Positive |
|
Mantoux test |
Negative |
|
TB Quantiferon gold test |
Negative |
|
Antistreptolysin O (ASO) titer |
Negative |
|
Lactate dehydrogenase (LDH) |
1054.0 U/L |
|
Serum ferritin levels |
292.29 ng/ml (Normal range: 4.63 to 204.00 ng/ml) |
|
ACE levels |
65.37 U/Lt (Normal range: 8.0 to 52.0 U/Lt) |
|
Liver function tests |
WNL |
|
Serum amylase |
WNL |
|
Serum electrolytes |
WNL |
* WNL- Within normal limits
ANA 1:320 +++, homogenous, and anti dsDNA was
positive. Antiphospholipid (APA)-IgG
antibody - 65.48 U/ml (negative < 10.00 U/mL);
APA-IgM antibody - 81.41 U/ml (negative <10.00
U/ml). The autoantibody profile (CCP-Ab, SS-A, SS-B,
Ro-52, Scl-70, PM-Scl, Jo-1, anti-mitochondrial Ab, PCNA, p-ANCA, c-ANCA) was negative. However, ribosomal
P-protein was weakly positive. LE cell was not detected and rheumatoid factor
was negative.
Skin biopsy showed an unremarkable epidermis, with dermis showing the
presence of perivascular lymphocytic infiltrate. The
presence of inflammatory infiltrate, composed of mainly lymphocytes, with few eosinophils and neutrophils, were
suggestive of lobular panniculitis. Presence of hyaline fat necrosis was a
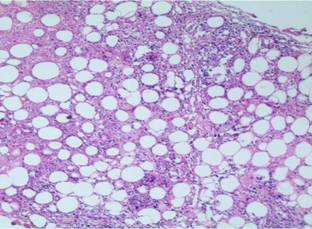
distinctive feature. There was no evidence of dermal edema or any granulomas (Fig 4).
Fig 4: Lobular
panniculitis with a predominantly lymphocytic infiltrate and hyaline fat
necrosis (H & E original magnification x 10)
Periodic acid Schiff and Gomori methenamine silver stains did not reveal any fungal elements. Negative Ziehl Neelsen and Leishman stains ruled out the presence of atypical mycobacteria and Leishmaniasis respectively. Direct immunofluorescence studies were negative and the immunohistochemistry results excluded the possibility of subcutaneous T-cell panniculitis. The presence of CD3-positive cells was noted. An increase in the number of CD8 T-cells was found when compared to CD4-positive T-cells. Bone marrow biopsy showed normocellular hematopoesis with no evidence of granuloma or malignancy. No abnormalities were noticed on conducting thorough ophthalmologic evaluation including slit lamp examination and fundoscopy. MRI was also done to rule out any retinal vaso-occlusive diseases, which have been reported rarely in complicated cases presenting with orbital inflammation in LEP.5
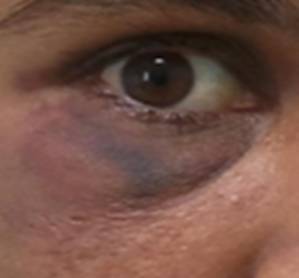
The final diagnosis was established as LEP and the patient was administered with intravenous methylprednisolone (1 g/day) for 3 consecutive days. Injecting intralesional triamcinolone acetonide 10 mg/ml into the right periorbital subcutaneous swelling contributed to its resolution in two weeks with no residual visual deficit or atrophy (Fig 5).
Fig 5:
Resolution of the right periorbital swelling post
treatment
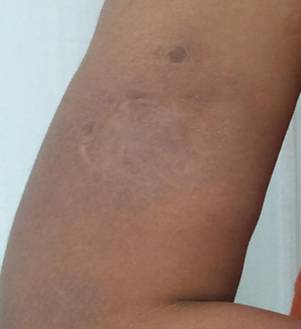
On discharge, the patient was prescribed with azathioprine 50 mg daily and prednisolone 40 mg daily. She was also instructed how to taper the steroid doses gradually. The subcutaneous nodules resolved completely in one month with atrophy and post-inflammatory hyperpigmentation (Figure 6). Occurrence of new lesions was not found.
Fig 6: Resolution
of the subcutaneous nodule with significant atrophy over the right upper arm
Although, the patient did not fulfill the American Rheumatism Association criteria for systemic lupus erythematosus (SLE), she was being closely monitored through routine investigations during follow-up.
Discussion
LEP, also known as Kaposi-Irgang lupus erythematosus profundus, is a rare skin condition originally described by Kaposi in 1883 and later by Irgang in1940.1 It is a clinical variant of LE primarily affecting the deep dermis and subcutaneous fat. It may occur on its own or along with discoid lupus erythematosus (DLE) or SLE. LEP is more prevalent in the age group of 20-60 years. It is very rare in children and only 12 fully reported prior cases are available in the English literature.2
The clinical presentation of LEP is characterized by the occurrence of erythematous nodules on the trunk, scalp, face and proximal extremities and the disease is usually treated using corticosteroids and hydroxychloroquine. Deep, saucerized depressions are generally produced by the attachment of overlying skin to the subcutaneous nodules. Lesions often heal with significant atrophy. There is mostly lobular panniculitis with inflammatory infiltrate of lymphocytes. The presence of lymphoid follicles in the subcutaneous fat, observed in majority of the cases, is the characteristic of LEP. But it has also been described in erythema nodosum panniculitis, dermatomyositis, morphea profundus, and subcutaneous T-cell lymphoma. Around 50% of the patients with LEP have evidence of SLE, but the systemic features tend to be less severe. Ophthalmic manifestations reported with SLE affect the visual system in 20% of individuals and must be ruled out with a thorough ophthalmologic evaluation. These include keratoconjunctivitis sicca, periocular skin lesions, orbital inflammation with secondary central retinal artery occlusion, retinal hemorrhages and vasculitis, retinal vaso-occlusive disease, iritis, scleritis, and optic neuropathy.3 Occasional case reports of ocular lesions as the solitary initial manifestation masquerading as idiopathic orbital inflammatory syndrome, highlights the need for a high index of suspicion for such presentations to diagnose LEP or SLE at an early stage.4 LEP should be considered in patients with a characteristic rash and orbital inflammation and may cause acquired enophthalmos.
The differential diagnosis would include connective tissue panniculitis such as panniculitis associated with dermatomyositis, morphea, and scleroderma-associated panniculitis. However, ulceration is a feature more commonly associated with lupus panniculitis or lupus profundus. Infective panniculitis comprises of cutaneous tuberculosis, atypical mycobacteria, subcutaneous fungal infections, leishmaniasis, and necrotic erythema nodosum leprosum. These can be differentiated using special stains, culture, and clinicopathological correlation.
The characteristic histopathological examination of LEP reveals a lobular panniculitis with deep lymphocytic infiltration, occasionally extending into the septa. A distinctive feature is the ‘hyaline necrosis’ of the fat in which portions of the fat lobule have lost nuclear staining of the fat cells and have an accumulation of fibrin and other proteins in a homogenous eosinophilic matrix between the residual fat cells and extracellular fat globules. Histopathologic differential diagnosis with subcutaneous panniculitis-like T-cell lymphoma may also be extremely difficult.
ANA was positive only in 27% of cases of LEP studied by Patrica et al.5 These patients were already diagnosed to have SLE or progression to SLE. This was lower than that previously reported by Winkelmann et al. (67% of 29 patients), Watanabe and Tsuchida (56% of 16 patients), and Martens et al. (65% of 40 patients).6, 7, 8 In the series reported by Watanabe and Tsuchida, more than half of the nine patients who were ANA positive, did not fulfill the criteria for SLE after a mean follow-up duration of 9.8 years.7 Similarly, the study by Martens et al reported that only four of the 26 patients who were ANA positive had SLE.8 These study findings substantiate that ANA positivity did not correlate with progression to SLE in the successive years.
Direct immunofluorescence (DIF) studies show a positive immunofluorescent band test with deposition at the dermal-epidermal junction and blood vessel walls, with IgM being the predominant immunodeposit and C3 and IgG seen in half of the cases. DIF was positive only in 36% of cases reported by Patricia et al compared to 70% reported by Sanchez et al.5, 9
Management of LEP is based largely on case series and expert opinion due to the lack of evidence from controlled therapeutic trials. Hydroxychloroquine and moderate dose corticosteroid are effective for the treatment of LEP. Case reports of successful treatment with dapsone are encouraging. Autologous fat transfer in patient with lupus erythematosus profundus is done for correction of cosmetic disfigurement such as hemifacial atrophy after resolution of facial lesions.
Generalized LEP has been reported in association with hereditary deficiencies of complement, particularly of C2 and C4, at young age with significant cutaneous involvement and Ro positivity.10 The patient in the current case study had normal complement levels and absence of anti-Ro antibodies. This excluded the possibility of having lupus associated with complement deficiencies.
Antiphospholipid syndrome (APS) is characterized by the
presence of recurrent arterial and venous thrombosis, recurrent foetal loss, mild to moderate
thrombocytopenia, lupus anticoagulant, and anticardiolipin
antibodies. The patient in the current case study was detected to have APA
positivity, persistent thrombocytopenia and anemia, but no prior history of any
fetal loss or thrombosis, hence did not fulfill the criteria for APS. LEP with
no evidence of systemic features of SLE may be associated with APA positivity
or APS. To our knowledge there are only few case reports of LEP associated with
APA: one patient with generalized LEP and APLA positivity, without complement
deficiency; a case of lupus mastitis with APS; and another patient who had
panniculitis and fasciitis with positive APA, but no APS.11, 12, 13
The association of APLA with LEP has been hypothesized to contribute to the elastolytic process, which leads to resolution with atrophy
in LEP.14
Conclusion
The present case study focuses on the unusual features of LEP in the form of generalized cutaneous involvement with initial solitary periorbital edema. Association of APLA with autoimmune hemolytic anemia and APLA positivity with no systemic features of SLE is also rare.
Competing interests
The
authors declare that they have no competing interests.
References
1.
Süss R, Meurer M,
Schirren CG, Lübke S, Ruzicka T. [Kaposi-Irgang lupus erythematosus profundus. Lupus erythematosus panniculitis]. Hautarzt.
1994 Jan;45(1):38-41.
2.
Weingartner JS, Zedek DC, Burkhart CN, Morrell DS. Lupus erythematosus panniculitis in children: report of three
cases and review of previously reported cases. PediatrDermatol.
2012 Mar-Apr;29(2):169-176.
3.
Sudhakar P, Shah GV, Saponara
F, Fullen DR, Trobe JD. Central retinal artery
occlusion secondary to orbital inflammation in lupus erythematosus
profundus. J Neuroophthalmol.
2012 Mar;32(1):93-94.
4.
Ohsie LH, Murchison AP, Wojno
TH. Lupus erythematosus profundus
masquerading as idiopathic orbital inflammatory syndrome. Orbit. 2012 Jun;31(3):181-183.
5.
Tan
S H, Tan T. Lupus erythematosus panniculitis: a clinicopathologic study.Int J Dermatol. 2002, 41 :488–490.
Patricia Pei-Lin Ng,
6.
Winkelmann RK. Panniculitis in
connective tissue disease. ArchDermatol1983;119:
336–344.
7.
Watanabe
T, Tsuchida T. Lupus erythematosus
profundus: a cutaneous marker for a distinct clinical
subset? BrJ Dermatol 1996;
134: 123–125.
8.
Martens
PB, Moder KG, Ahmed I. Lupus panniculitis: clinical
perspectives from a case series. J Rheumatol 1999;
26: 68–72.
9.
Sanchez
NP, Peters MS, Winkelmann RK. The histopathology of
lupus erythematosus panniculitis. J Am Acad Dermatol 1981; 5: 673–680.
10.
Provost
TT, Arnett FC, Reichlin M. Homozygous C2 deficiency,
lupus erythematosus and anti-Ro (SSA) antibodies.
Arthritis Rheum 1983;26:1279–1282.
11.
Nousari HC, Kimyai-Asadi
A, Santana HM, Diglio GM, Tausk
FA, Cohen BA. Generalized lupus panniculitis and antiphospholipid
syndrome in a patient without complement deficiency. Pediatr
Dermatol. 1999 Jul-Aug;16(4):273-276.
12.
Devine DV, Brigden
ML. The antiphospholipid syndrome: When does the
presence of antiphospholipid antibodies require
therapy? Postgrad Med 1996;99:105–108.
13.
Carsuzaa F, Pierre C, De Jaureguiberry
JP, Marlier S, Morand JJ, Marrot F. Syndrome panniculite-fasciite
revelant un lupus erythemateux
systemique. Ann Dermatol Venereol 1996;123:259–261.
14.
Marzano A, Vanotti
M, Alessi E. Anetodermic
lupus panniculitis and antiphospholipid antibodies:
report of three cases. Acta Derm
Venereol. 2004;84(5):385-8.